Published on 05.03.2021
Understanding genetic deafness
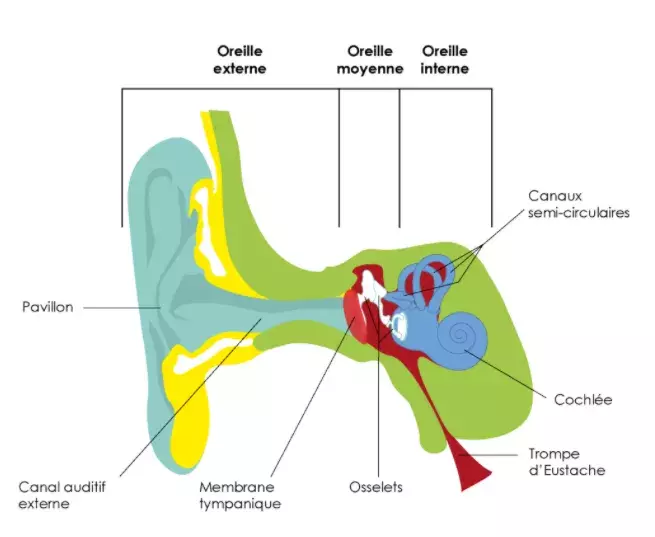
The ear, a complex functioning
Our ears are made up of three main parts: the outer ear, which contains the ear canal, the middle ear, which amplifies sound, and the inner ear, which plays a major role in our hearing and balance.
The cochlea and vestibule, both located in our inner ear, contain sensory cells that pick up sound vibrations from the outside and transform them into electrical signals. As they pass through the auditory nerves, the electrical signals produced by the sensory cells in the cochlea are then processed by the brain as sounds and that is how we hear.
Damage to any of these parts of the ear can cause different forms of hearing loss of varying severity.
Not one, but several genetic deafness
Deafness can be divided into three main categories:
- Conductive deafnesss related to damage to the outer and/or middle ear.
- Perceptive or neurosensory deafness, related to the cochlea, and/or the auditory nerve pathways, and/or the central structures of hearing.
- Mixed deafness, combining these two components.
80% of early deafness not related to ear infections are genetic in origin. Hearing loss can exist from birth or appear later in life until adulthood.
"It can be difficult to identify the genetic causes of deafness because abnormalities in several different genes can lead to the same type of deafness, whereas identical genes can cause different deafness depending on how they are altered: a mutation in a gene can, for example, cause isolated (non-syndromic) deafness, whereas another mutation in the same gene can cause syndromic deafness, i.e. accompanied by other symptoms," explains Sylvain Ernest, a researcher at Institut Imagine.
Approximately 200 genes responsible for non-syndromic deafness have been discovered to date, and 500 syndromic deafnesses have been described, which may be associated with kidney, eye, neurological and cardiac disorders in particular.
For more information on deafness, reference centers, care, patient associations, go to the site of the rare diseases expertise platform of Hôpital Necker-Enfants malades AP-HP and Filière SensGène.
Genetic deafness, which path of research and care for patients?
To improve the diagnosis and management of these patients, research and healthcare teams are working to understand the multiple causes of these deafnesses and hope to be able to find treatments. They are proposing a research and care pathway that works in a loop and places patients at the center of the system. This virtuous loop starts from the particular case of each patient and returns to him or her.
The consultation, the first step in the patient's journey
At Imagine, everything starts with the patient. Children or adults suffering from genetic deafness generally consult with Dr. Sandrine Marlin, who runs the genetic deafness reference center at the Hôpital Necker-Enfants malades AP-HP, affiliated with Institut Imagine.
A committed, passionate doctor, the daughter of a mother who was a professor at the National Institute for Deaf Children, she has always been surrounded by people who are deaf or hard of hearing. She has been deeply involved since her master's degree at the Pasteur Institute, and in 1995, she created the world's first clinical genetics consultation dedicated to deafness of genetic origin. In the 2000s, she set up the first network bringing together ENT specialists, clinical geneticists and specialized molecular biologists. She manages this national network which, in 2004, was labeled by the Ministry of Health as "centers of reference for rare diseases".
In these consultations, Dr. Sandrine Marlin offers multidisciplinary care with a psychologist, a nurse, and a genetic counselor. This care is provided in direct collaboration with the ENT team and a team of specialized biologists (Dr Laurence Jonard). Communication methods during consultations are adapted to each family. The pediatric site of the Genetic Deafness Reference Center works in collaboration with a specialized team at the Pitié Salpêtrière Hospital (ENT; Specialized Midwife) to allow an adapted transition from child to adult.
Approximately 1/3 of the consultations concern adults and 2/3 of the children, seeking above all to know the cause of their deafness. During this consultation, Dr. Marlin will try to establish a diagnosis and a cause by analyzing one or more genes already known to cause deafness. If no mutation is identified during these tests, a research study can be proposed in the Imagine associate laboratory.
2nd step: research takes over to support medicine
In the laboratory of embryology and genetics of malformations, directed by Jeanne Amiel at Imagine, Dr. Sandrine Marlin and Dr. Sylvain Ernest seek to identify new genes involved in deafness. Depending on how the gene is altered, it may cause different types of deafness, isolated or syndromic. The research team at Imagine is primarily dedicated to congenital deafness, which appears at birth or early childhood, and in particular to sensorineural deafness, with malformations and/or syndromes.
To find the cause of a patient's deafness, researchers identify mutations in candidate genes by studying the so-called coding part of DNA, the part that translates into proteins. In particular, they study whether a mutation produces an abnormal protein that exists in the ear; whether this protein is involved in the development or functioning of the ear; and whether this damage is found in other families. If no abnormality is found in the part of a gene that is transformed into a protein, the search for abnormalities may extend to other elements that have an impact on protein expression and regulation and may therefore also be responsible for deafness.
More than 200 genes have already been identified worldwide as the cause of syndromic deafness. Several of them have been identified in the Imagine Institute's laboratory and many projects are underway, with leads on genes that have not yet been implicated in deafness.
3rd step: from research to the patient, the diagnosis
If the research team is able to identify a new gene responsible for the patient's deafness, it then goes back to the participating families to let them know the cause of their deafness.
These scientific advances will then benefit other patients by being integrated into the genetic tests offered in consultation. The identification of a new gene can therefore make it possible to provide a diagnosis to other patients in the waiting list but also to future patients with the same genetic anomaly. Identifying the cause of deafness makes it possible to improve medical care (indications for certain hearing aids; advice and care for future pregnancies; prognosis; prevention, etc.). These discoveries also contribute to a better understanding of how hearing works in general and to the search for treatments.
Once the diagnosis has been made, the management of patients
To date, there are different types of multidisciplinary care available, but there is no treatment yet. However, the results of research conducted at Imagine suggest that treatments will be available in the future.
The research team dedicated to developmental abnormalities of the ear at Imagine, in collaboration with the ENT department of Hôpital Necker-Enfants malades AP-HP and the Institut de l'Audition, is already working on a hospital-university research project (RHU) aimed at developing a first gene therapy for a type of profound deafness in children.