Published on 04.03.2026
Presentation
Research Topics
Click a topic to jump to section:
- General Investigation: Genetic factors shaping vulnerability and resilience to neurological diseases
- Shared Mechanisms: Metabolic Disorders & Neurodegeneration
- The Gut-Brain Axis: Immune Defense & Neuronal Integrity
- Advanced Models: Organoids & Organ-on-Chip
Investigating how genetic factors shape vulnerability and resilience to neurological diseases.
We are particularly interested in the mechanistic links between rare inherited disorders and common age-related neurodegenerative diseases. By uncovering fundamental biological pathways shared across these conditions, we aim to identify convergent mechanisms that drive brain dysfunction and ultimately reveal new therapeutic opportunities. Our research centers around three main directions:
Understanding shared mechanisms between monogenic metabolic disorders and complex neurodegenerative diseases.
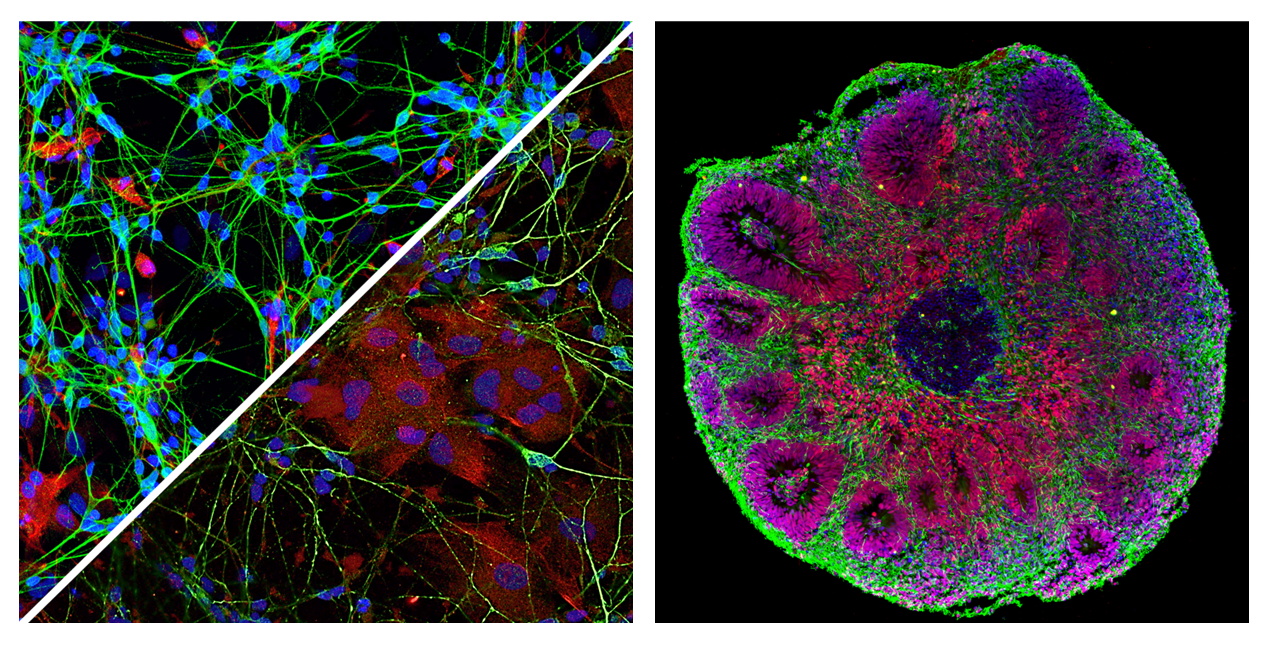
We study how mitochondrial diseases, lysosomal storage disorders, and other rare genetic conditions intersect with the pathophysiology of Alzheimer’s and Parkinson’s diseases. Mitochondria represent a central theme in our work. We investigate how mitochondrial dysfunction contributes to neuronal vulnerability, immune dysregulation, and altered intercellular communication in neurodegeneration.
By integrating genetic models of mitochondrial disorders with tricultures or organoid platforms and patient-derived iPSCs, we dissect how impaired energy metabolism, defective mitophagy, and maladaptive mitochondrial stress responses drive pathological changes shared across rare and common brain diseases. These insights help explain why specific neuronal and glial populations are selectively affected in neurodegeneration.
Exploring the gut–brain–immune axis in neurodegeneration.
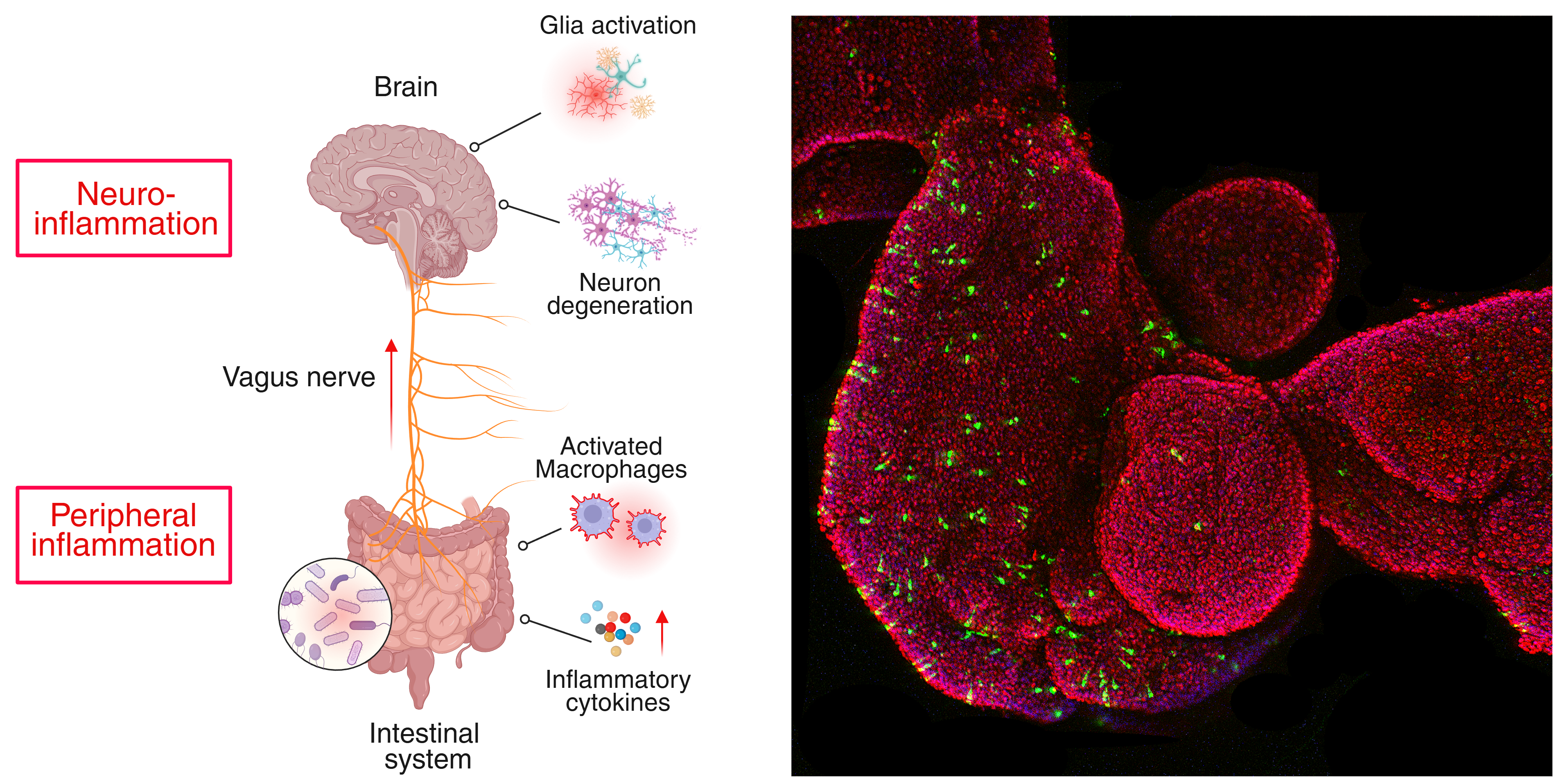
We examine why disorders that appear unrelated, such as inflammatory bowel disease or chronic infections, display epidemiological and mechanistic links to Parkinson’s disease. In this context, we aim to define how genetic traits influence host–pathogen interactions and shape long-term brain health. We investigate how variants that enhance resistance to infection may paradoxically predispose individuals to neurodegeneration later in life, revealing trade-offs between immune defense and neuronal integrity.
Developing advanced human model systems to study disease mechanisms.
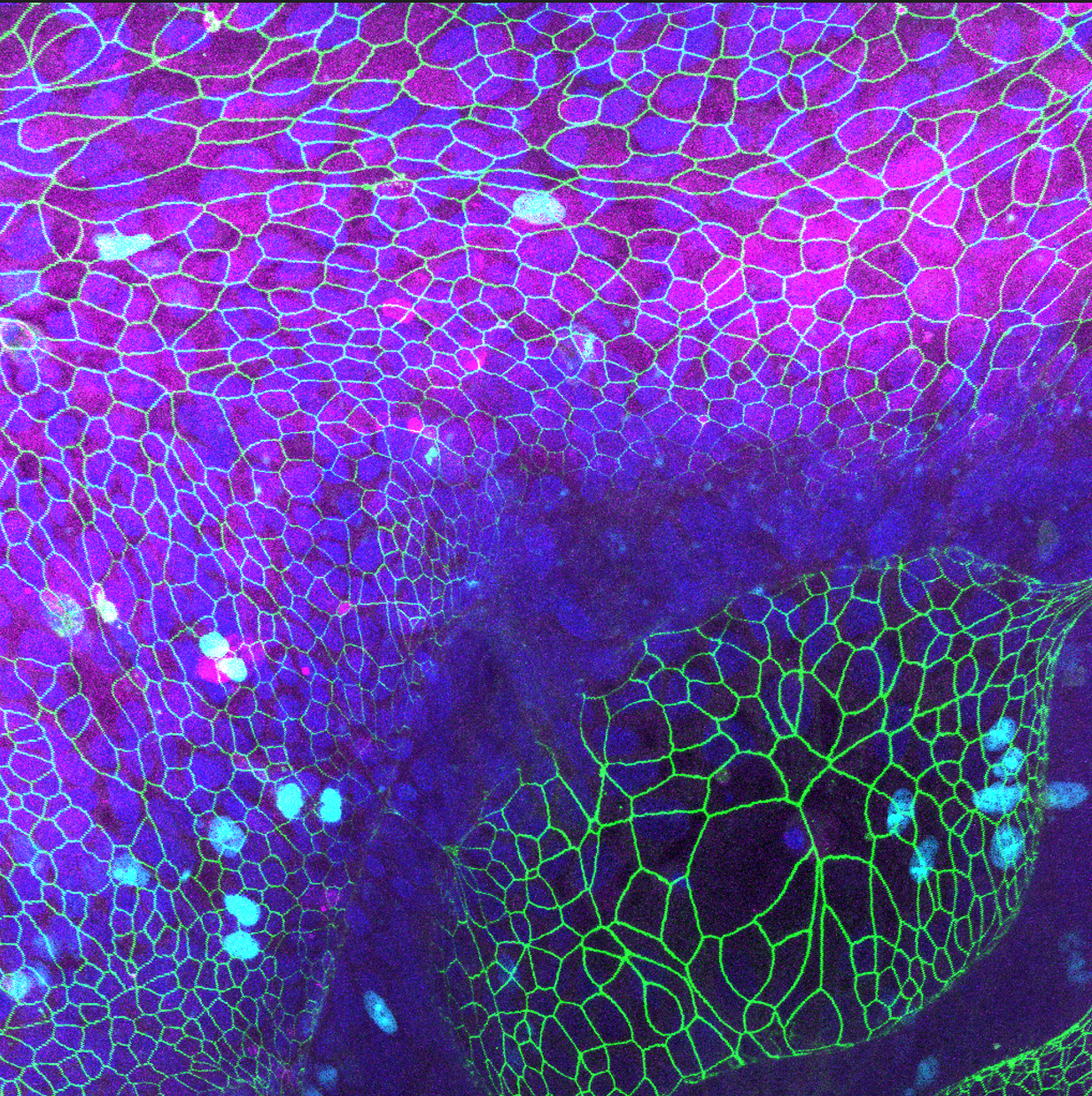
A major technological focus of our lab is the generation of next-generation human models, including brain organoids and multi-organ systems. We engineer long-term brain organoids that recapitulate key aspects of human cortical and midbrain development, enabling the study of neuronal maturation, microglial function, and early disease-associated changes with high fidelity.
In parallel, we are establishing gut–brain organ-on-chip platforms that integrate intestinal epithelium, microbiome components, immune cells, and neural tissues within dynamic microfluidic circuits. These systems allow us to investigate bidirectional communication between the gut and the brain, model environmental exposures such as pesticides, and uncover mechanisms linking peripheral inflammation, metabolic stress, and neurodegeneration.
To address our biological questions, we combine these human model systems with induced pluripotent stem cell (iPSC) technologies, single-cell transcriptomics, functional imaging, and computational approaches. This integrated strategy enables us to dissect cellular and molecular mechanisms underlying neurodegeneration across development, aging, and environmental influence.










