Published on 23.10.2025
Presentation

Contact
Human genetics of infectious diseases : Mendelian predisposition
Our team aims to determine the molecular basis of the monogenic determinism of rare and common infectious diseases in children. We hypothesize that a substantial fraction children with severe infectious diseases suffer from novel single-gene inborn errors of immunity, resulting in a specific susceptibility to one or a few microorganisms. During the last years, we have provided further evidence supporting this hypothesis, with the discovery of the molecular genetic basis of:
- The syndrome of Mendelian predisposition to mycobacterial disease (MSMD), severe pediatric tuberculosis, and syndromic forms of mycobacterial disease, due to mutations of IFN-g immunity. We discovered new recessive etiologies of mycobacterial disease, in patients carrying specific mutations of: 1) ISG15 in patients with mycobacterial disease and auto- immunity, 2) TYK2 in patients with mycobacterial and viral infections, and 3) RORC in patients with mycobacterial and fungal infections.
- Invasive pneumococcal disease (IPD) due to mutations in the NF-kB pathway. Following on our identification of NEMO, IRAK4, and MyD88 deficiencies, we identified the first patients with impaired linear ubiquitination, due to mutations in HOIL- 1 or HOIP, two components of the LUBAC. These patients have auto-inflammation and bacterial infections. Linear ubiquitination via LUBAC is thus essential for the modulation of inflammation and the control of bacterial infections.
- Life-threatening influenza due to IRF7 deficiency. The amplification of anti-viral IFNs is IRF7-dependent in both plasmacytoid dendritic cells (PDCs) and induced pluripotent stem cell (iPSC)-derived pulmonary epithelial cells. Herpes simplex encephalitis (HSE) due to inborn errors of TLR3 immunity. The pathogenesis of HSE involves the impairment of IFN production by neurons and oligodendrocytes in the central nervous system.
- Epidermodysplasia verruciformis (EV), due to papillomaviruses, and Kaposi sarcoma (KS), due to human herpes virus 8. Both conditions can result from inborn errors of T cell immunity (mutations in RHOH and MST1 for EV, and mutations in STIM1 and OX40 for KS). Other acute viral conditions, such as fulminant viral hepatitis, are also being studied.
- Fungal diseases, such as chronic mucocutaneous candidiasis disease (CMCD) and deep dermaphytosis disease (DDD).
CMCD results from inborn errors of IL-17 immunity (loss-of- function mutations in IL17F, IL17RA and ACT1, and gain-of-function mutations in STAT1) and DDD from bi-allelic null mutations in CARD9. Invasive fungal diseases, such as cryptococcosis and aspergillosis, are also being studied.
These projects are based on a worldwide recruitment of patients, and a cutting-edge strategy combining genome- wide investigations, in particular using next-generation sequencing, with in-depth functional studies of leukocyte subsets or iPSC-derived non-hematopoietic cells. Overall, our work provides proof-of-principle that severe infectious diseases in otherwise healthy children and young adults, in the course of primary infection, may result from single-gene inborn errors of immunity that rarely display
complete penetrance. This provides a model for the genetic architecture of severe infectious diseases. Our studies also showed that certain immunological pathways play a relatively narrow role in protective immunity to infections in natural conditions, at odds with the mouse model of experimental infections.
Team
To read also
-
Research Acceleration
09/07/2025
InFlaMe project: addressing virus-host interactions and defense strategies to design new therapeutics against flaviviruses
-
Research Acceleration
21/10/2024
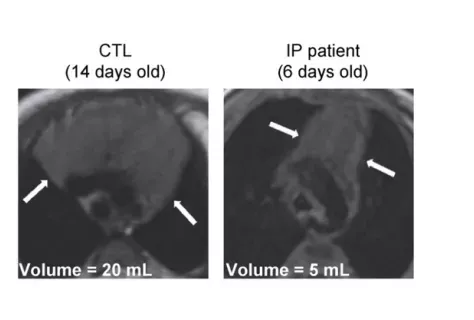
Incontinentia Pigmenti, a rare skin disease that also predisposes to viral infections
-
Research Acceleration
28/08/2024
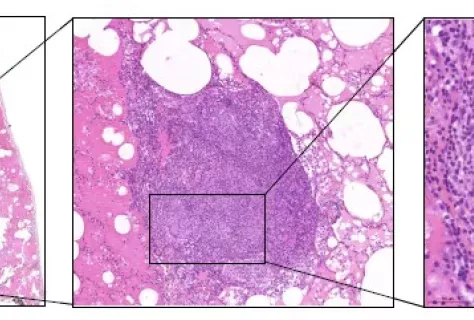
The first inherited TNF deficiency in human underlies susceptibility to tuberculosis
-
Research Acceleration
03/05/2024
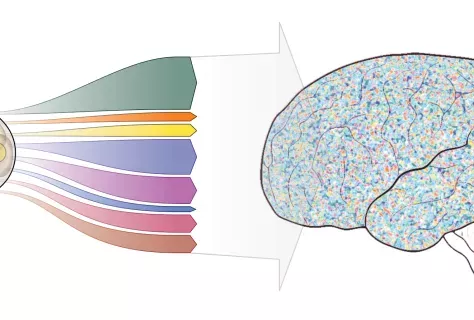
The role of dendritic cells in papillomavirus infections: from the FLT3LG gene to therapeutic perspectives
-

Research Acceleration
06/03/2024
Discovery of a frequent genetic cause of autoimmunity From rare to frequent variants: the case of pre-TCRα deficiency
Resources & publications
-
 2020Journal (source)Science
2020Journal (source)ScienceInborn errors of type I IFN immunity in patients with life-threatening COVID-19.
-
 2020Journal (source)Science
2020Journal (source)ScienceAuto-antibodies against type I IFNs in patients with life-threatening COVID-19.
-
 Journal (source)Proc. Natl. Acad. Sci. U.S.A.
Journal (source)Proc. Natl. Acad. Sci. U.S.A.Common homozygosity for predicted loss-of-function variants reveals both redu...
-
 2020Journal (source)J. Clin. Invest.
2020Journal (source)J. Clin. Invest.Inherited human IFN-γ deficiency underlies mycobacterial disease.
-
 2019Journal (source)J. Clin. Invest.
2019Journal (source)J. Clin. Invest.Rescue of recurrent deep intronic mutation underlying cell type-dependent qua...
-
 2019Journal (source)J. Exp. Med.
2019Journal (source)J. Exp. Med.Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reacti...
-
 2019Journal (source)Nat. Med.
2019Journal (source)Nat. Med.Human SNORA31 variations impair cortical neuron-intrinsic immunity to HSV-1 a...
-
 Journal (source)Proc. Natl. Acad. Sci. U.S.A.
Journal (source)Proc. Natl. Acad. Sci. U.S.A.Homozygosity for TYK2 P1104A underlies tuberculosis in about 1% of patients i...
-
 2019Journal (source)Front Immunol
2019Journal (source)Front ImmunolCDG: An Online Server for Detecting Biologically Closest Disease-Causing Gene...
-
 2019Journal (source)Hum. Mol. Genet.
2019Journal (source)Hum. Mol. Genet.A purely quantitative form of partial recessive IFN-γR2 deficiency caused by ...
-
 2019Journal (source)J. Exp. Med.
2019Journal (source)J. Exp. Med.Inherited IL-18BP deficiency in human fulminant viral hepatitis.
-
 Journal (source)Proc. Natl. Acad. Sci. U.S.A.
Journal (source)Proc. Natl. Acad. Sci. U.S.A.Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form ...
-
 2019Journal (source)J. Exp. Med.
2019Journal (source)J. Exp. Med.Severe influenza pneumonitis in children with inherited TLR3 deficiency.
-
 2019Journal (source)Curr. Opin. Immunol.
2019Journal (source)Curr. Opin. Immunol.Human inborn errors of immunity to infection affecting cells other than leuko...
-
 2019Journal (source)Bioinformatics
2019Journal (source)BioinformaticsPopViz: a webserver for visualizing minor allele frequencies and damage predi...
-
 2019Journal (source)J. Clin. Immunol.
2019Journal (source)J. Clin. Immunol.A Variety of Alu-Mediated Copy Number Variations Can Underlie IL-12Rβ1 Defici...
-
 2019Journal (source)J. Clin. Immunol.
2019Journal (source)J. Clin. Immunol.Autosomal Dominant IFN-γR1 Deficiency Presenting with both Atypical Mycobacte...
-
 Journal (source)Proc. Natl. Acad. Sci. U.S.A.
Journal (source)Proc. Natl. Acad. Sci. U.S.A.Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form ...
-
 2019Journal (source)J. Clin. Invest.
2019Journal (source)J. Clin. Invest.Inherited p40phox deficiency differs from classic chronic granulomatous disease.
-
 2019Journal (source)Sci Immunol
2019Journal (source)Sci ImmunolTuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous...
-
 2019Journal (source)Sci Immunol
2019Journal (source)Sci ImmunolHuman IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23.
-
 2019Journal (source)J. Exp. Med.
2019Journal (source)J. Exp. Med.The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to...
-
 2019Journal (source)J. Exp. Med.
2019Journal (source)J. Exp. Med.Life-threatening influenza pneumonitis in a child with inherited IRF9 deficie...
-
 Journal (source)Proc. Natl. Acad. Sci. U.S.A.
Journal (source)Proc. Natl. Acad. Sci. U.S.A.A deep intronic splice mutation of STAT3 underlies hyper IgE syndrome by nega...
-
 2019Journal (source)Nucleic Acids Res.
2019Journal (source)Nucleic Acids Res.SeqTailor: a user-friendly webserver for the extraction of DNA or protein seq...
-
 2019Journal (source)Nat. Immunol.
2019Journal (source)Nat. Immunol.Disruption of an antimycobacterial circuit between dendritic and helper T cel...
-
 2019Journal (source)Sci Immunol
2019Journal (source)Sci ImmunolA recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT...
-
 Journal (source)Proc. Natl. Acad. Sci. U.S.A.
Journal (source)Proc. Natl. Acad. Sci. U.S.A.Blacklisting variants common in private cohorts but not in public databases o...
-
 2019Journal (source)Cell
2019Journal (source)CellInborn Errors of RNA Lariat Metabolism in Humans with Brainstem Viral Infection.
-
 Journal (source)Proc. Natl. Acad. Sci. U.S.A.
Journal (source)Proc. Natl. Acad. Sci. U.S.A.Incomplete penetrance for isolated congenital asplenia in humans with mutatio...
-
 2018Journal (source)PLoS Negl Trop Dis
2018Journal (source)PLoS Negl Trop DisMicrodeletion on chromosome 8p23.1 in a familial form of severe Buruli ulcer.






