Publié le 25.02.2026
Présentation
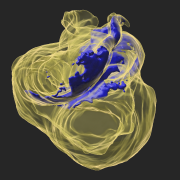
Le laboratoire Imagine-Institut Pasteur de Morphogenèse du Coeur s'intéresse aux mécanismes embryonnaires de la formation du coeur. Nos recherches fondamentales dans le modèle murin ont des applications potentielles dans le domaine médical, pour les malformations congénitales et la réparation du cœur.
Projet scientifique
La forme d'un organe est étroitement liée à sa fonction. Pour le coeur, l'architecture du myocarde conditionne la fonction de contraction, et l'alignement des chambres cardiaques la double circulation sanguine.
Sans sculpteur ni ingénieur, comment les cellules s’assemblent-elles pour façonner un organe ? Comment connaissent-elles leurs positions relatives dans un tissu ? Comment leurs comportements sont-ils coordonnés au cours du développement embryonnaire pour générer une forme ?
Nous répondons à ces questions en utilisant des technologies de pointe en génétique, omiques, imagerie 3D, analyse quantitative de données complexes et simulations par ordinateur. Nous travaillons en étroite collaboration avec le Centre de Référence des Malformations Cardiaques Congénitales Complexes (M3C).
Equipe
Ressources & publications
-
 2025Journal (source)Sci Adv
2025Journal (source)Sci AdvPlasticity of ventricle position after heart looping in heterotaxy with right...
-
 2025Journal (source)PLoS Biol
2025Journal (source)PLoS BiolNotch3 is an asymmetric gene and a modifier of heart looping defects in Nodal...
-
 2023Journal (source)Dev Cell
2023Journal (source)Dev CellIdentification of Greb1l as a genetic determinant of crisscross heart in mice...
-
 2021Journal (source)Annu Rev Gen Hum Genet
2021Journal (source)Annu Rev Gen Hum GenetHeart Development and Congenital Structural Heart Defects.
-
 2020Journal (source)Dev Cell
2020Journal (source)Dev CellTransient Nodal Signaling in Left Precursors Coordinates Opposed Asymmetries ...
-
 2017Journal (source)Elife
2017Journal (source)ElifeA predictive model of asymmetric morphogenesis from 3D reconstructions of mou...